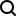Reverse Osmosis-A complete Guide
Click Here to See How a Reverse Osmosis System Works
Anyone who has been through a high school science class will likely be familiar with the term osmosis. The process was first described by a French Scientist in 1748, who noted that water spontaneously diffused through a pig bladder membrane into alcohol. Over 200 years later, a modification of this process known as reverse osmosis allows people throughout the world to affordably convert undesirable water into water that is virtually free of health or aesthetic contaminants. Reverse osmosis systems can be found providing treated water from the kitchen counter in a private residence to installations used in manned spacecraft.
Reverse Osmosis is a technology that is found virutally anywhere pure water is needed; common uses include:
- Drinking Water
- Humidification
- Ice-Making
- Car Wash Water Reclamation
- Rinse Waters
- Biomedical Applications
- Laboratory Applications
- Photography
- Pharmaceutical Production
- Kidney Dialysis
- Water used in chemical processes
- Cosmetics
- Animal Feed
- Hatcheries
- Restaurants
- Greenhouses
- Metal Plating Applications
- Wastewater Treatment
- Boiler Water
- Battery Water
- Semiconductor production
250 PPM
Before using R/O System
(TDS range from 250-450ppm)
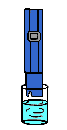
10 PPM
After using R/O System
(TDS range from 5-26 ppm)
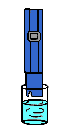
A semipermeable membrane, like the membrane of a cell wall or a bladder, is selective about what it allows to pass through, and what it prevents from passing. These membranes in general pass water very easily because of its small molecular size; but also prevent many other contaminants from passing by trapping them. Water will typically be present on both sides of the membrane, with each side having a different concentration of dissolved minerals. Since the water i the less concentrated solution seeks to dilute the more concentrated solution, water will pass through the membrane from the lower concentration side to the greater concentration side. Eventually, osmotic pressure (seen in the diagram below as the pressure created by the difference in water levels) will counter the diffusion process exactly, and an equilibrium will form.
Distillation is an Alternative to Reverse Osmosis Water Treatement
Distillation involves boiling the water to produce water vapor. The water vapor then rises to a cooled surface where it can condense back into a liquid and be collected. Because the dissolved solids are not normally vaporized, they remain in the boiling solution.Distillation is one of mankind's earliest forms of water treatment, and it is still a popular treatment solution throughout the world today. In ancient times, the Greeks used this process on their ships to convert sea water into drinking water. In far-eastern cultures, water was distilled for use in "Ranbiki" tea ceremonies.
Today, distilled water is still used to convert sea water to drinking water on ships and in arid parts of the world, and to treat water in other areas that is fouled by natural and unnatural contaminants. Distillation is perhaps the one water treatment technology that most completely reduces the widest range of drinking water contaminants.
Not only is distillation one of the most effective forms of treatment, but it is also one of the easiest to understand: untreated water is converted into water vapor, which is then condensed back into liquid form. Most of the contaminants are left behind in the boiling chamber, with the condensed water being virtually contaminant-free. Anyone who has accidentally let a pot of water boil completely out on the stove is familiar with this process, and familiar with the crust of contaminants typically left behind after the water is gone.
In nature, this basic process is responsible for the hydrologic cycle. The sun causes water to evaporate from surface sources such as lakes, oceans, and streams. The water vapor eventually comes in contact with cooler air, where it re-condenses to form dew or rain. This process can be imitated artificially, and more rapidly than in nature, using alternative sources of heating and cooling.
Water is passed between a positive electrode and a negative electrode. Ion selective membranes allow the positive ions to separate from the water toward the negative electrode and the negative ions toward the positive electrode. High purity de-ionized water results. The water is usually passed through a reverse osmosis unit first to remove nonionic organic contaminants.
Deionization (DI) is a water filtration process whereby total dissolved solids (TDS) are removed from water through ion exchange. In simple terms, by controlling the electric charge of ions in the water, it is possible to remove the TDS. Much like a positively charged magnet will attract a negatively charged magnet (and vice-versa), DI resins attract non-water ions and replace them with water ions, leaving a more pure water form.
The process of deionization uses two resins that are opposite in charges – the cationic (negative) and the anionic (positive). The cationic resin is typically made from styrene containing negatively charged sulfonic acid groups, and will be pre-charged with hydrogen ions. This resin will attract the positively charged ions in the water (Ca++, Mg++, Na+, etc.) and releases an equivalent amount of hydrogen (H+) ions.
Like the cationic, the anionic resin is also made from styrene, but contains positively charged quaternary ammonium groups, and will be pre-charged with hydroxide ions. This resin will attract the negatively charged ions (HCO3-, Cl-, SO4--, etc.) and releases an equivalent amount of hydroxide (OH-). The hydrogen and hydroxide ions then combine to form water. (H+ + OH- = HOH or H2O.)